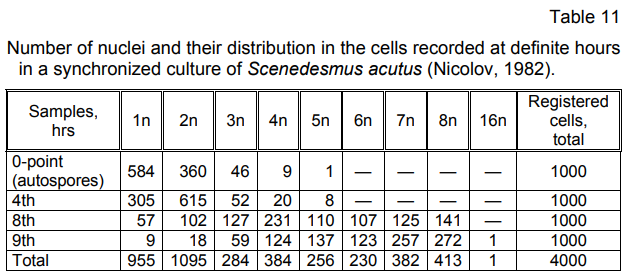
Page 202
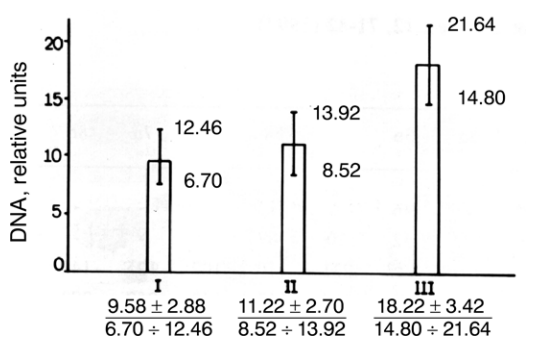
Figure 3–26. Diagram of results of cytophotometric measuring of DNA-amount in cells of Scenedesmus acutus treated with bisbenzimide H-33258.
The asynchronous division is also confirmed by the data of flow
cytometry. Recently this method finds a successful application in studying
the dynamics of quantitative changes of DNA during individual phases of
life cycle in different species of suspensial cell cultures. What is more,
registration of such processes related with cell division is difficult to be
realized by other research methods.
The histograms of a synchronized cell culture of Scenedesmus acutus
(Fig. 3–27, 1—5) outline a fluent, consecutive process of quantitative
changes of DNA at fixed hours of the life cycle, which corresponds to a
consecutive (asynchronous) development and division of the nuclei,
observed on the photoregistered cytological cell pictures. The absence of
clearly expressed peaks only at exactly fixed moments (phases), that would
mark nuclear division in two, four, eight, etc., demonstrates that nuclear
reproduction occurs asynchronously, consecutively, which correlates with
the results obtained using the other research methods.