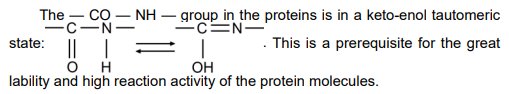
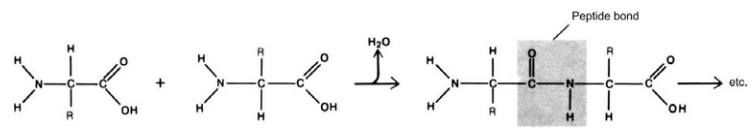
Figure 2–31. Binding of the amino acids by peptide bonds and formation of polypeptide chains.
Protein molecules configuration is determined by several levels of
organization. The amino acid sequence in the polypeptide chain with
NH₂-group unengaged at the one end and the COOH-group at the other
is called primary structure (Fig. 2–32).
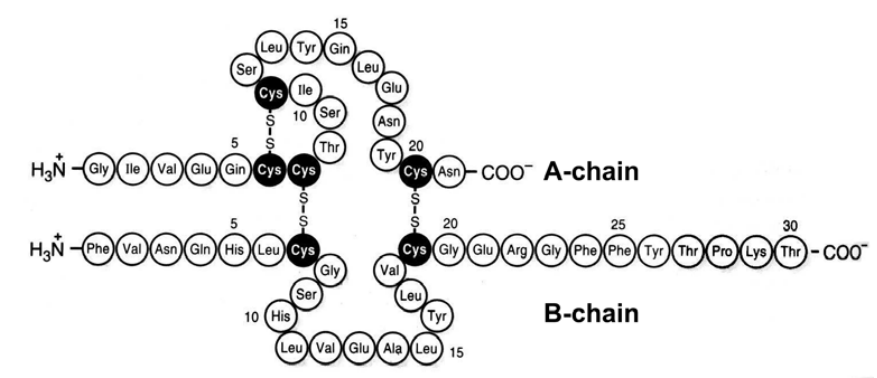
Figure 2–32. Primary structure of human insulin. Disulphide bridges of the native molecule are shown.
Each protein molecule has a spatial configuration as well, determined
by secondary (α-spiral configuration of the polypeptide chains due to the
formation of hydrogen bonds), tertiary (coiling of the chains owing to the
interactions of the amino acid residues inside the chains) and quaternary
(characterized by the formation of bonds between the amino acid residues
from different polypeptide chains) levels of organization determining in their
part its biological activity. These are presented in Figures 2–33 A and B.