Depending on the number of carbon atoms they are trioses, tetroses,
pentoses, hexoses, heptoses and octoses. All monosaccharides, called
also simple sugars, are colourless crystal substances easily soluble in
water, most of them sweet to the taste.
Natural monosaccharides (with the exception of dihydroxyacetone)
possess optical activity expressed in turning polarized light to the right or to
the left. This phenomenon is due to the asymmetric carbon atoms in their
molecules. For example the triose glyceraldehyde has three carbon atoms,
and only one of them is asymmetric. It exists in two different stereoisomers
— D-right and L-left form (see Fig. 1–1). A hexose with four asymmetric
carbon atoms can have 16 isomers (2⁴=16).
The pentoses ribose and deoxyribose (Fig. 2–48) participate as
components in the nucleotides — the basic building block of the RNA and
DNA molecules. That determines their biological importance compared to
the other monosaccharides.
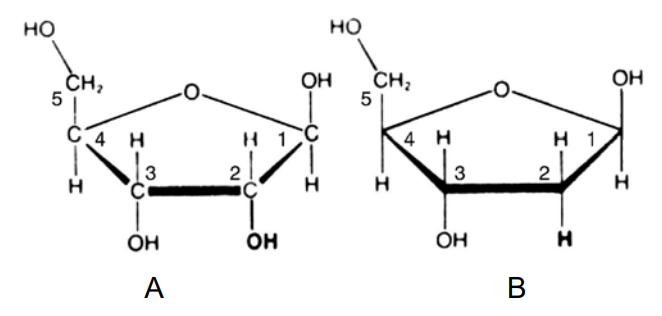
Figure 2–48. Structural formulas of the pentoses ribose and deoxyribose, participating in nucleic acids. A — β-D-ribose, a component of RNA; B — β-D-2-deoxyribose, a component of DNA.
Among the hexoses glucose and fructose (Fig. 2–49) are most spread
in nature. Both sugars have the same formula (C⁶H¹²O⁶), but the one is
aldose, and the other — ketose. Glucose is the main product of
photosynthesis. As a result of a number of consecutive redox processes
during the dark phase it is transformed into different carbohydrate
derivatives used to meet the energetic needs of the cells and in
synthesizing different organic components (see Chapter 1, Metabolism).