was mainly due to CO₂ and N₂, actively participating in the metabolic
processes.
Carbon remains the most “mysterious” element. It forms stable bonds
and chains of various configurations, different sizes and functional groups.
The availability of four electrons in its outer envelope is the prerequisite for
the formation of four covalent bonds with the atoms of other elements as
well as with its own
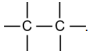
The bonds of carbon atom with hydrogen ones are very stable. From
a biological point of view especially important are the peptide bonds
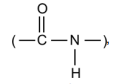
which lead to the formation of long polypeptide chains
resulting from the release of H₂O from the carboxyl group of one of the amino
acids and the amino group of the other one (see Figs. 2–31 and 2–52). The
combination of these four organic elements and the linkage of amino acids in
polypeptides with the release of water can be regarded as the beginning of the
origin of life supplied with simpler mechanisms of self-reproduction without the
participation of nucleic acids.
A serious “candidate” for the place and the role of carbon in the build-up
of living entities could be silicon (Si). It ranks second in content in the
lithosphere (21.2%) right after oxygen. It also has four valencies. According
to Raubach (1983) two or more molecules of ortho-silicic acid
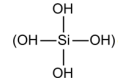
can bind with the release of H₂O and thus give rise to
higher acids forming in this process chain, bunch-like and spatial-network
structures. For unknown reasons silicon has “preferred” to remain in a
bound state as a main component of onyx, quartz, achate and silicon
dioxide (SiO₂) or sand — most widely spread on the earth surface.
Sulphur and phosphorus join company at a later stage. Phosphorus
takes an active part in the structure of nucleic acids, which assume the
function of bearers of genetic information and participate in the replication
mechanisms of living creatures.
The increase of oxygen content in the earth atmosphere as a result
mainly of photosynthesis has drastically changed metabolism of living
organisms. The anaerobic release of energy from glucose of 47 kcal is
replaced by the aerobic one in which the released energy is several times
greater — 686 kcal. All possible sources of energy have been subjected to