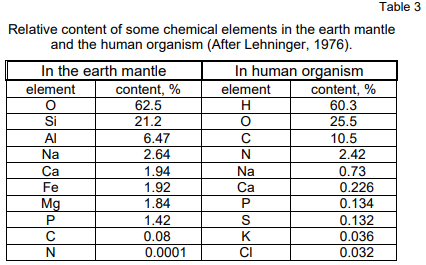
element with the highest content in the earth mantle — 62.5% (see Table 3)
has not taken an active part in the exchange of substances since it was in a
bound state. Hydrogen, water and carbon dioxide have played the primary
role in these early processes.
If we however have a look at the chemical formulae of the basic
biomolecules — amino acids and nucleic acid bases (see Figs. 2–29 and
2–37 A), we shall got easily convinced that alongside carbon, hydrogen and
nitrogen, oxygen also takes part in them.
The question crops up: where has oxygen come from? It could have
come from the compounds in which it has been bound or from water and
carbon dioxide. The second assumption seems more plausible.
Most obviously oxygen has been “prevented” from taking part in the
primeval metabolic process. Hydrogen was entirely another case. This is
the lightest chemical element with a high reaction capacity and has been
found in various physical states: nascent atoms (H), ions (H+), as well as
bound to other atoms. That is why it has actively participated in the
synthesis of biomolecules and the metabolic processes. Its content in the
human organism is the highest — 60.3%. It is no wonder that the hydrogen
ion concentration i.e. pH is of such an importance for the life processes and
chemical reactions in the cells. The oxygen content in human organism is
also high — 25.5%. This can be assumed to be due to its later accumulation
in a free state (O₂) in the atmosphere as a result mainly of photosynthesis.
The reverse tendency is observed with carbon and nitrogen. From
minimal quantities in the lithosphere these two elements have reached high
percentage in the human organism (10.5% and 2.42% respectively). This