by some analogues of pyrimidine and purine bases. These analogues
possess a structure very similar to that of usual bases — adenine, guanine,
cytosine and thymine. 5-bromouracil and 2-aminopurine are the best
studied among them.
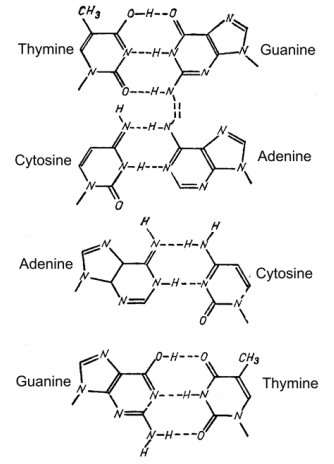
Figure 2–77. “Prohibited” nucleotide pairs, which arise when some base is in a rarely met tautomeric form. On the left — bases underwent the tautomeric transition. On the right — bases in the form usually met in DNA (After Rukmansky et al., 1984).
5-bromouracil (BU) is an analogue of thymine, in which the 5-
position methyl group (—CH₃) is substituted for brom atom (Br).
Normally, thymine and BU (in keto-form) bind adenine (T—A or A—BU).
When BU is in the rarely met enol-form, it forms hydrogen bonds with
guanine (G—BU). 2-aminopurine (AP) is an analogue of adenine and
should form a nucleotide pair with thymine (T—AP). If it is in imino-form,
it binds cytosine (C—AP) — Figure 2–78. Incorporating the base
analogues into the molecule of DNA leads to mistakes in
complementation with other bases which, on its part, causes replication
mistakes because of the realized transition AT ⇄ GC.