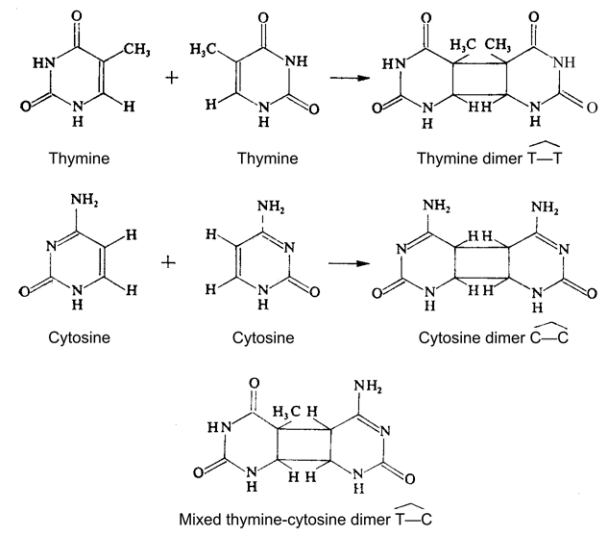
Figure 2–80. Pyrimidine dimers.
The mutation changes in chromosomes can be highly varied — from
changes in nucleotide composition and mistakes in binding the bases in
DNA to great structural transformations in them. These changes are
caused by different reasons, a lot of them being unclear. Some of them are
the already mentioned ionizing radiations and chemical mutagens. Among
the latter a special place is held by the alkylating compounds, some of them
known as supermutagens (Table 9).
The mechanisms of action of alkylating compounds are complex. They
cause various genetic changes — transitions, transversions, chromosome
aberrations, etc. It is accepted that these substances express their
mutagenic effect in two different ways. The one is a direct formation of
“incorrect” nucleotide pairs, and the other — alkylation of DNA-bases at
different positions through free radicals, like methyl (⎯CH₃), ethyl (⎯C₂H₅),
propyl (⎯C₃H₇), etc. In vitro alkylation has shown a preference to guanine
in 7th position (Lawley, 1966), while in vivo — great is the role of oxygen
atoms in 4th position for thymine and in the 6th for guanine (Auerbach,
1976; Singer, 1976).